How to Determine Which Electrode Works Best for a Redox.couple
Redox couples are analogous to conjugate acid-base pairs and behave in much the same way. The iodidetriiodide I I 3 redox couple is almost universal as the electrolyte in conventional DSSCs and has proven to be most effective for achieving high cell efficiencies.

Electrode Potential And Redox Reactions Calculations Uses Videos Q A
We have the demi-reaction of Oxidation Since the standard potential of the Copper couple is higher than the Manganese one.

. E E X 0 006 2 l o g 1. 50 First to avoid any possible complications from charging such as impurities. Now for the electrode potential.
The SAM with a photoactive group and an electron relay group formed on a metal electrode in a solution containing an electron acceptor andor donor should be a good candidate for a stable. The performances of electrodialysis ED and reverse electrodialysis RED processes depend on several factors including the nature of the electrode material and of the redox couple adopted to make possible the conversion between electric power and chemical potential. M n 2 O H X M n O H X 2 X 2 e X.
Carefully place the electrodes in the beaker and attach the alligator clips from the potentiostat to the electrodes so they are not in electrical contact with one another. In order to determine the potential of the reference electrode one would normally use a redox couple in the solution that has a known formal potential and. UnpackinG Remove the grey shock-absorbing plastic net and inspect the microelec-trode visually.
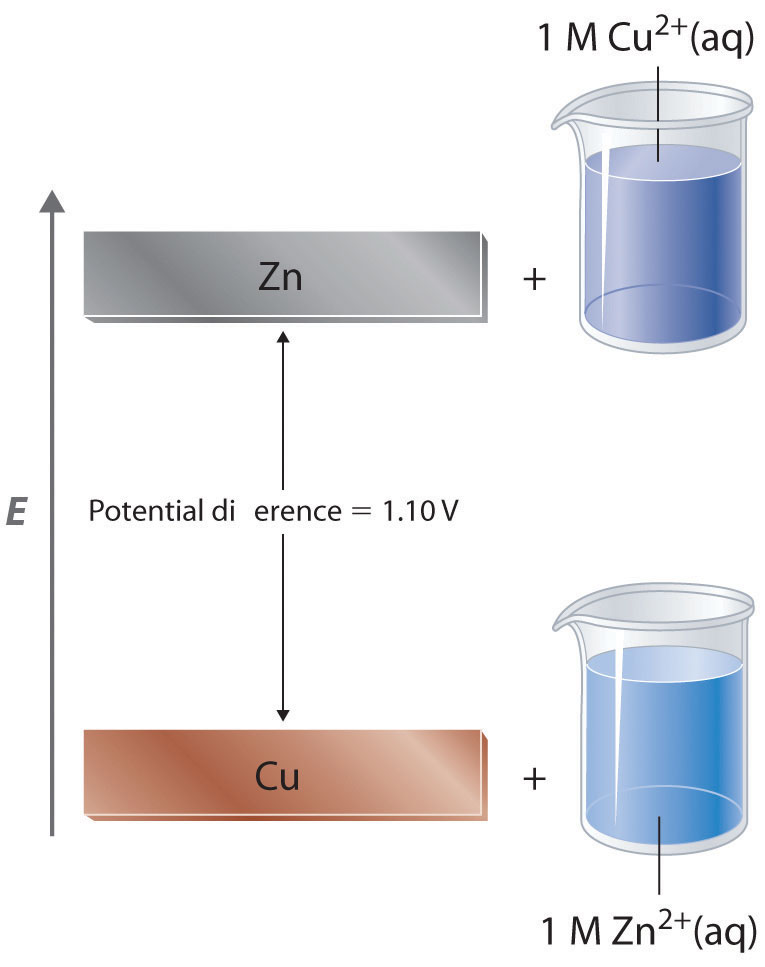
Determine the parameters and potential range necessary to observe the complete redox. The redox potentials for the various ferric. The potential of a half-reaction measured against the SHE under standard conditions is called the standard electrode potential for that half-reactionIn this example the standard reduction potential for Zn 2 aq 2e Zns is 076 V which means that the standard electrode potential for the reaction that occurs at the anode the oxidation of Zn to Zn 2 often called.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Write a balanced equation for both the reduction reaction and the oxidation reaction. Working out the cell potential for a proposed redox reaction makes it possible to suggest whether that reaction would be feasible.
The magnesium has the greater amount of negativeness - the voltmeter records that as negative. To do this I am using an ceAgAgCl electrode connected to an inert cePt electrode which is submerged in a solution containing ceFe3 ions. The voltmeter records it as positive.
In this paper the possible utilization of iron-based redox couples FeCl 3 FeCl 2. The stronger an oxidizing agent the weaker the. The standard reduction potential values are from an experiment made with a voltaic cell with the other electrode being SHE Standard Hydrogen Electrode.
The tendency of any chemical substance to lose or gain electrons is called the redox potential and is measured in volts V. See the answer See the answer See the answer done loading. Fe aq 3ox2 Feoxs E0770 V Feox1 a K17x 105 20x 1020 a A -0212 V B 0121 V C 121 V D -0121 V E121V.
Throughout the whole of this redox potential work you have to think in relative terms. Popoff and Kunz studied the influence of the variation of iron concentration and acid concentration on measured potentials from which the redox potential was determined to be 07477 V. It has a suitable redox potential E 0305 V SHE depending on the solvent V SHE is the potential relative to standard hydrogen electrode and acceptable light absorption in the visible region.
Bray and Hershey corrected for the hydrolysis and complex chloride formation and reported the electrode potential to be 0772 V. If you have a metal like Zn Cu. Use a table of Standard Electrode Potentials Standard Reduction Potentials to find the value of E o for both reactions.
E oreduction for the reduction reaction. Determine the standard electrode potential for the following redox couple FeoxaqFeox aq where oxC204 oxalate Given. Selected Redox Couples Arranged in Order of Decreasing Strength of Oxidizing Agent.
The value for H is assigned with a value of 0 V. I then titrate it with ceCo2 which reduces it to ceFe2The reference and inert electrode. The platinum of the hydrogen electrode isnt as negative - it is relatively more positive.
The reverse of the reaction the oxidation has the cell potential opposite to the sign of the reaction cell potential of the other reaction. Fe3 aq e- t- Fe2 aq Fe2 aq 30x2. Leave the microelectrode in the protection tube for testing.
To determine if observed decay occurs because of electrode fouling or some other process on the electrode itself we employ the ferroceneferrocenium redox couple as a model system which is stable enough to be used as a reference redox event in nonaqueous electrolytes. This problem has been solved. An oxidizing and reducing agent which appear on opposite sides of a half-equation constitute a redox couple.
An inert electrode is needed to conduct the electrons but it is not part of the redox reaction. Im trying to perform a potentiometric titration to determine the redox potential of a ceFe3Fe2 couple. 3 Set-up EC-Lab to perform a two-step experiment consisting of an OCV followed by a CV.
Connect the redox electrode to the amplifier Connect the reference electrode to the connector on the redox electrode cable. Measurements are made using an electrode that has been standardized against the H 2-2 H couple whose redox potential is set at 00 under standard conditions pH 00 1M H 25C and 1 atmosphere pressure. You only need an inert electrode like Pt or C gr if your reactants cant function as an electrode like I2 and Ce ions.

Electrode Potential And Redox Reactions Calculations Uses Videos Q A
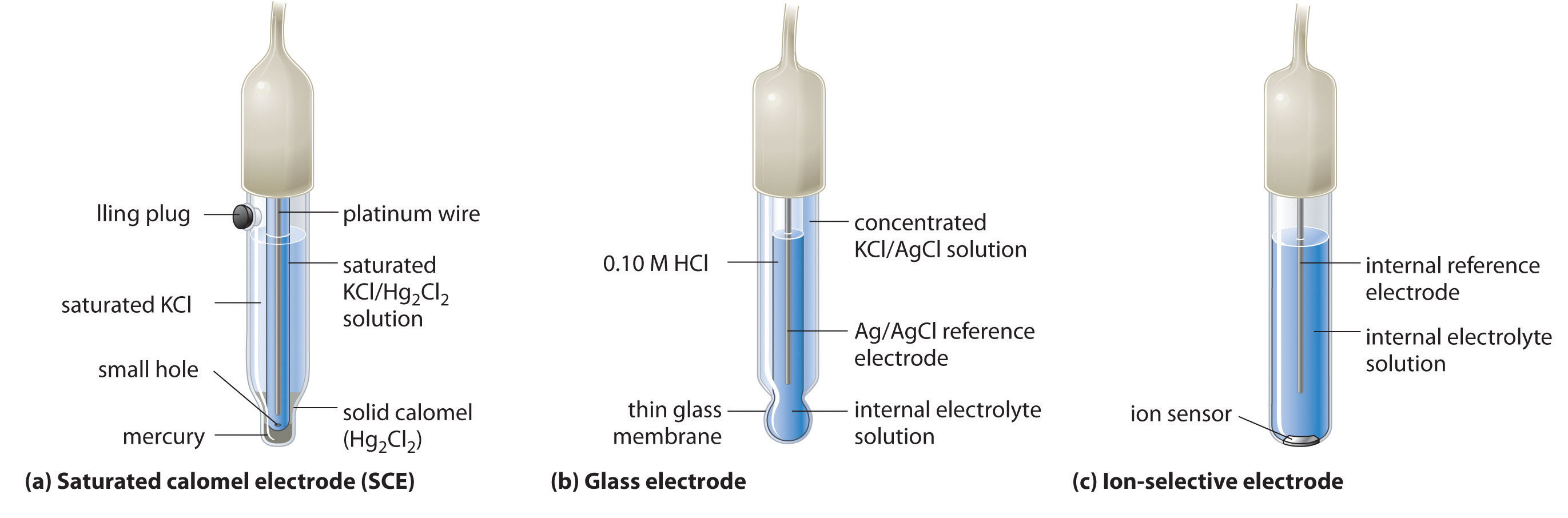
20 2 Standard Electrode Potentials Chemistry Libretexts

Comments
Post a Comment